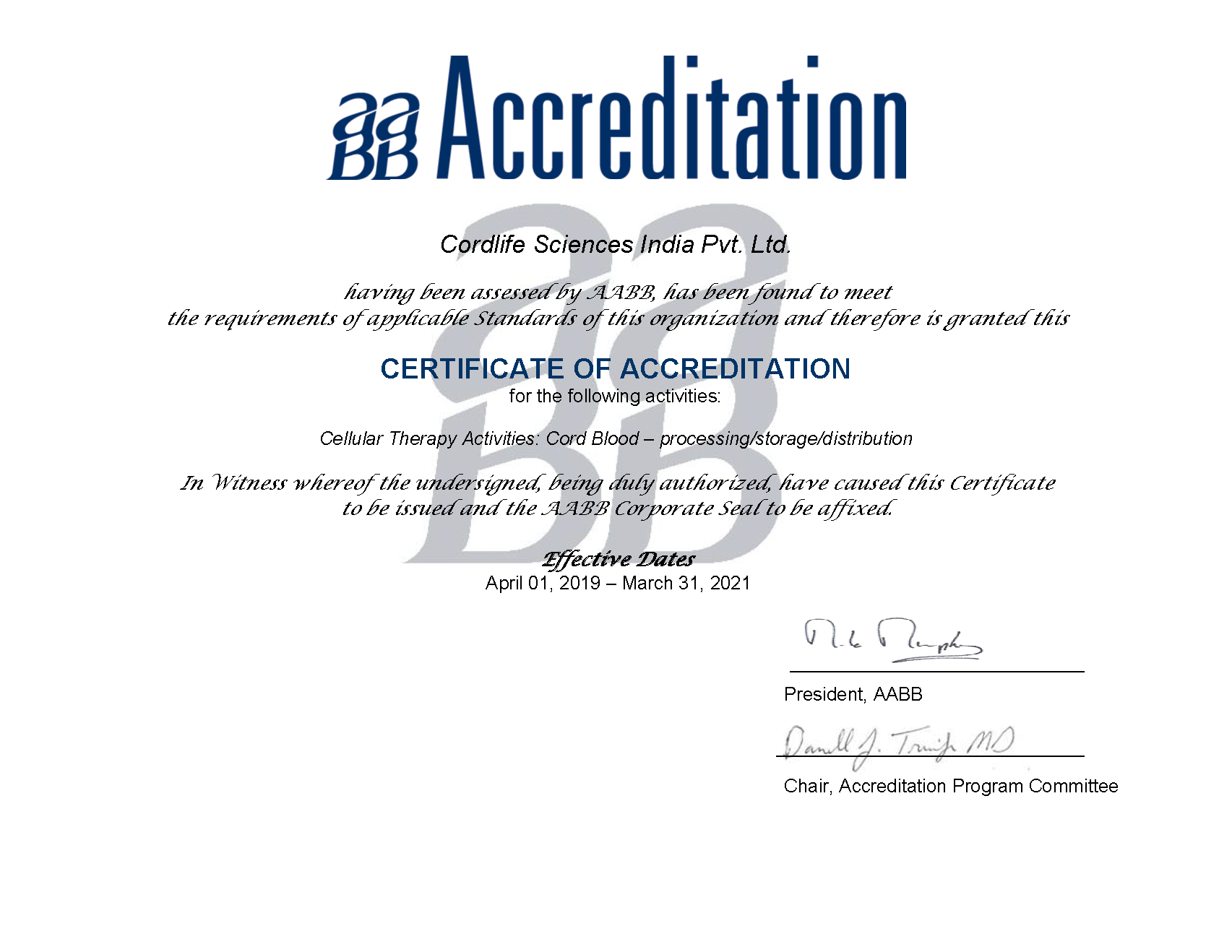
Internationally recognized and accepted accreditations ensure that the company has been assessed by independent peer experts for having the competence, reliability, operational performance and quality management of the processing and cryopreservation protocols to ensure patient safety at the time of transplant.
Along with our associates, Cordlife Group's in-depth knowledge of the business is recognised by many world-class gold standards and accrediting bodies such as the AABB, Foundation For The Accreditation Of Cellular Therapy (FACT), College of American Pathologists (CAP) and numerous government regulators. Such stringent quality system helps ensure that your baby’s stem cells are carefully processed and cryopreserved using rigorous protocols.
Along with our associates, Cordlife Group's in-depth knowledge of the business is recognised by many world-class gold standards and accrediting bodies such as the AABB, Foundation For The Accreditation Of Cellular Therapy (FACT), College of American Pathologists (CAP) and numerous government regulators. Such stringent quality system helps ensure that your baby’s stem cells are carefully processed and cryopreserved using rigorous protocols.





















Subscribe to our newsletter